Cutting-edge technology for breast cancer screening
Diagnostic-as-a-Service
that women need
Pain-free, low-cost, sensitive, radiation-free device to detect breast cancer in urine
The Problem
1 In 8 Women
will have breast cancer
at least once
BC is the most treatable cancer
but women, specially those with dense breast tissue, do not have reliable
screening methods available
It still remains the #1 cancer killer for women
81% of them want to get tested
for breast cancer
Proof of concept data
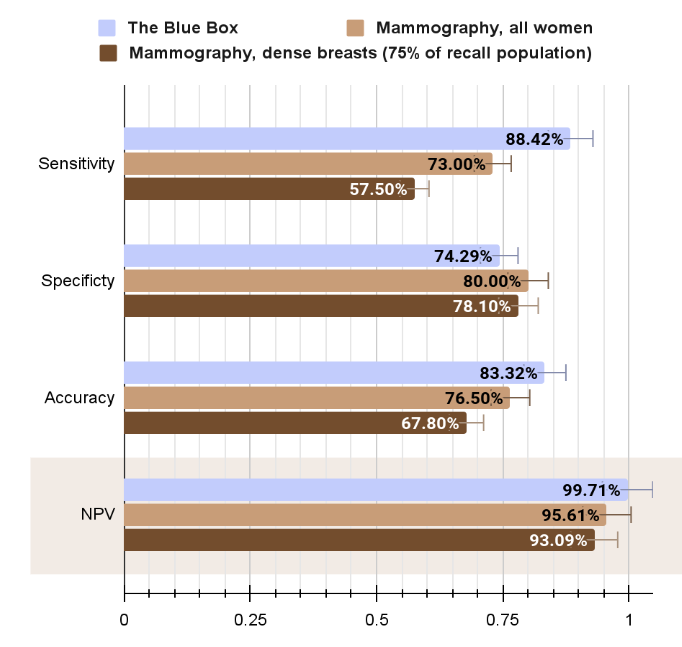
- "The Blue Box" (all women)
- Mammography (women <50)
Sensitivity
Accuracy
Specificity
Sensitivity
Accuracy
Specificity
- Sensitivity x1.5 higher than mammogram → less breast cancer patients left undiagnosed
- High Specificity → less unnecessary 2nd assessments (biopsies, higher-radiation dose mammographies, additional radiological exams…)
Our
Mission
Women aged 20-49 cannot get screened for breast cancer because existing solutions are unreliable. However, they want to, and are ready to pay for it. At “The Blue Box”, we have built a pain-free, low-cost, sensitive, radiation-free device to detect breast cancer in urine with a sensitivity of 88.33%.
Our
Mission
Breast cancer is the most treatable cancer, yet it remains the #1 cancer killer for women, as 50% are detected too late. At The Blue Box, we’ve built a urine-based breast cancer test with a sensitivity of 88.42%, outperforming mammography by 15%-30%. We’ll run clinical trials in 2025 and launch in gynecology clinics by 2026.

















During 3 years, we've collaborated with 7 hospitals, with over 450 women participating in our study

The Blue Box - Featured at















Key Opinion Leaders are Supporting Us

diagnosis in the world”

Join The Blue Box Family!
Do you want to be the first to know when The Blue Box will hit the market?
Subscribe to our newsletter and never miss out on The Blue Box journey!